Group leader
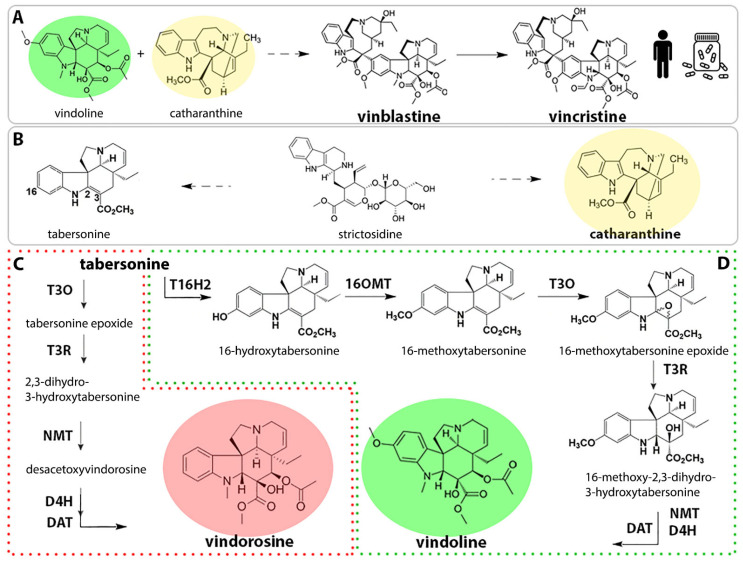
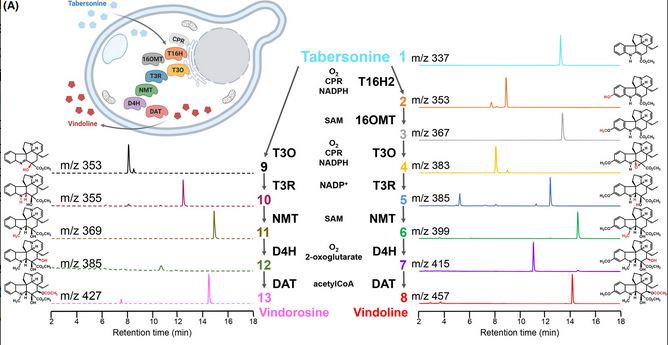
Highlight on one of our last publications
Read our publications
Follow the link here
Funded projects
► COMBO : a Horizon Europe project
Innovative cultivation method for biodiscovery
Communiqué de presse COMBO -2024

► MIAMi
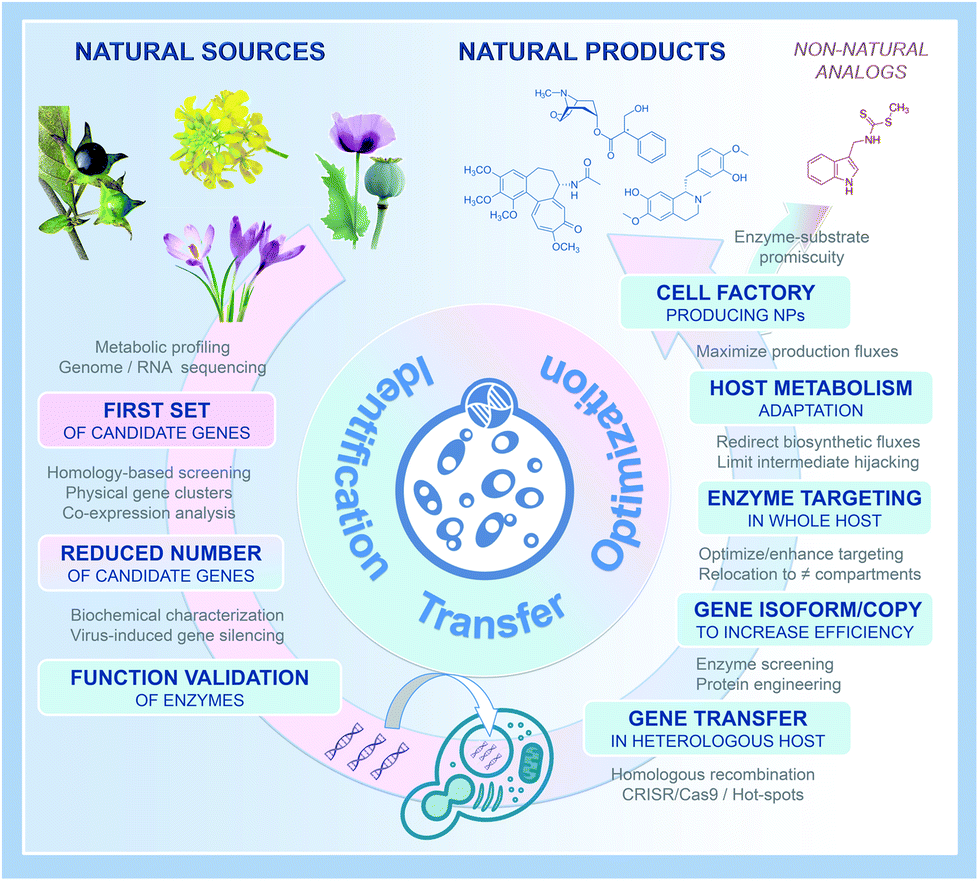
Refactoring MIA biosynthesis : an EU H2020 project

► MIACYC
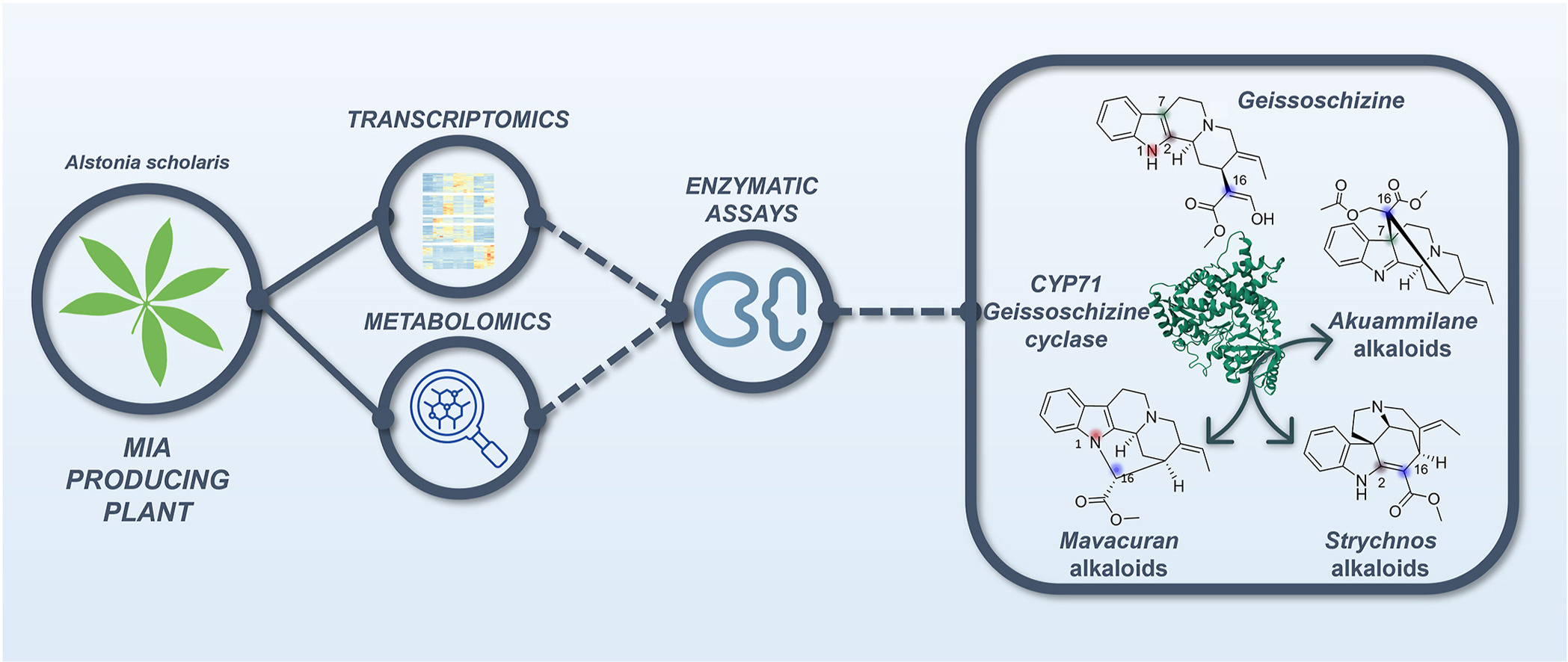
Unveiling the machinery driving atypical cyclisation of monoterpenoid indole alkaloids for metabolic engineering : an ANR project