Evaluation of type-B RR dimerization in poplar: A mechanism to preserve signaling specificity?
- Recherche,
A collaborative research article published in Plant Science, with the LBLGC team from the University of Orléans.
SW
Authors : I Djeghdir , F Chefdor , L Bertheau, K Koudounas, I Carqueijeiro, P Lemos Cruz, V Courdavault, C Depierreux, M Larcher, F Lamblin, F Héricourt, G Glévarec, A Oudin, S Carpin
DOI: 10.1016/j.plantsci.2021.111068
Abstract
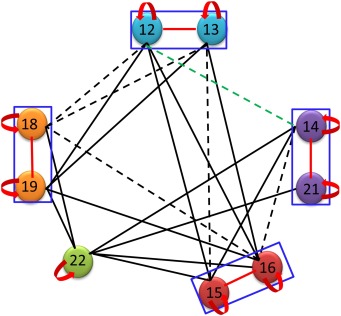
Plants possess specific signaling pathways, such as the MultiStep Phosphorelay (MSP), which is involved in cytokinin and ethylene sensing, and light, drought or osmotic stress sensing. These MSP comprise histidine-aspartate kinases (HKs) as receptors, histidine phosphotransfer (HPts) proteins acting as phosphorelay proteins, and response regulators (RRs), some of which act as transcription factors (type-B RRs). In previous studies, we identified partners of the poplar osmosensing signaling pathway, composed of two HKs, three main HPts, and six type-B RRs. To date, it is unresolved as to how cytokinin or osmotic stress signal specificity is achieved in the MSP in order to generate specific responses. Here, we present a large-scale interaction study of poplar type-B RR dimerization. Using the two-hybrid assay, we were able to show the homodimerization of type-B RRs, the heterodimerization of duplicated type-B RRs, and surprisingly, a lack of interaction between some type-B RRs belonging to different duplicates. The lack of interaction of the duplicates RR12-14 and RR18-19, which are involved in the osmosensing pathway has been confirmed by BiFC experiments. This study reveals, for the first time, an overview of type-B RR dimerization in poplar and makes way for the hypothesis that signal specificity for cytokinin or osmotic stress could be in part due to the fact that it is impossible for specific type-B RRs to heterodimerize.
Keywords: Dimerization; MultiStep phosphorelay; Populus; Type-B response regulators.
